 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?  Thank you for choosing ACROBiosystems. Would you rate our product and service?
Thank you for choosing ACROBiosystems. Would you rate our product and service?
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
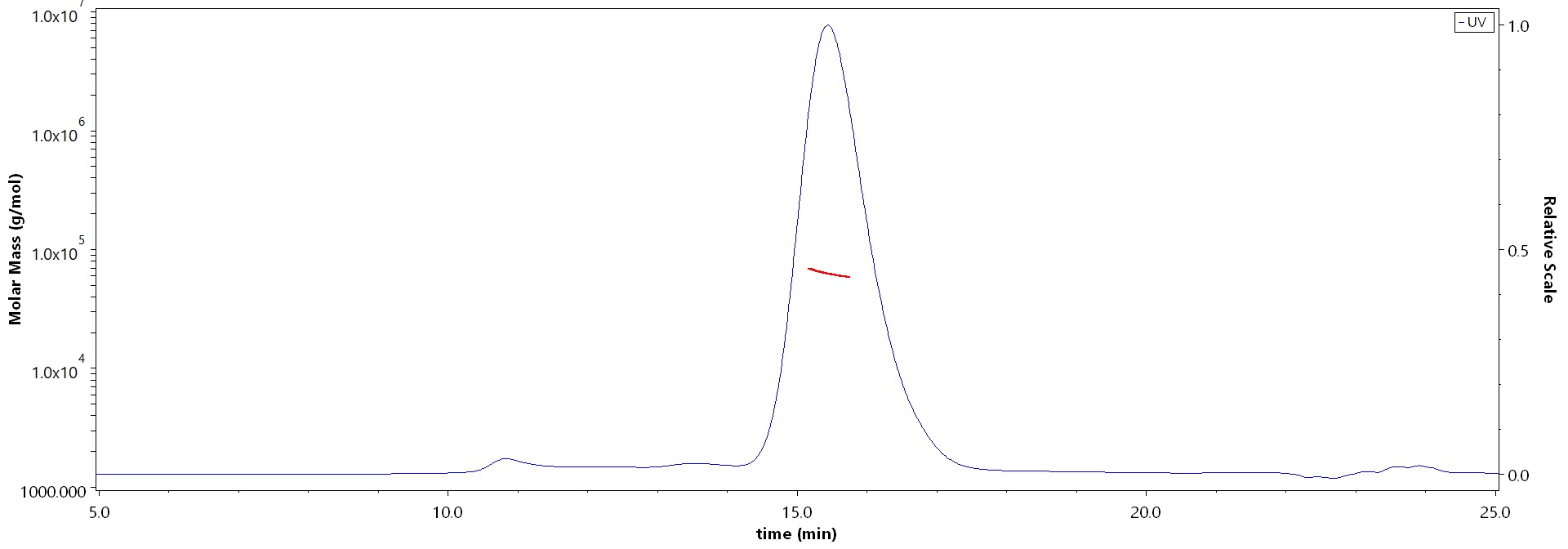
The purity of Human Coagulation factor IX Protein, His Tag (Cat. No. FIX-H52H3) is more than 90% and the molecular weight of this protein is around 55-70 kDa verified by SEC-MALS.
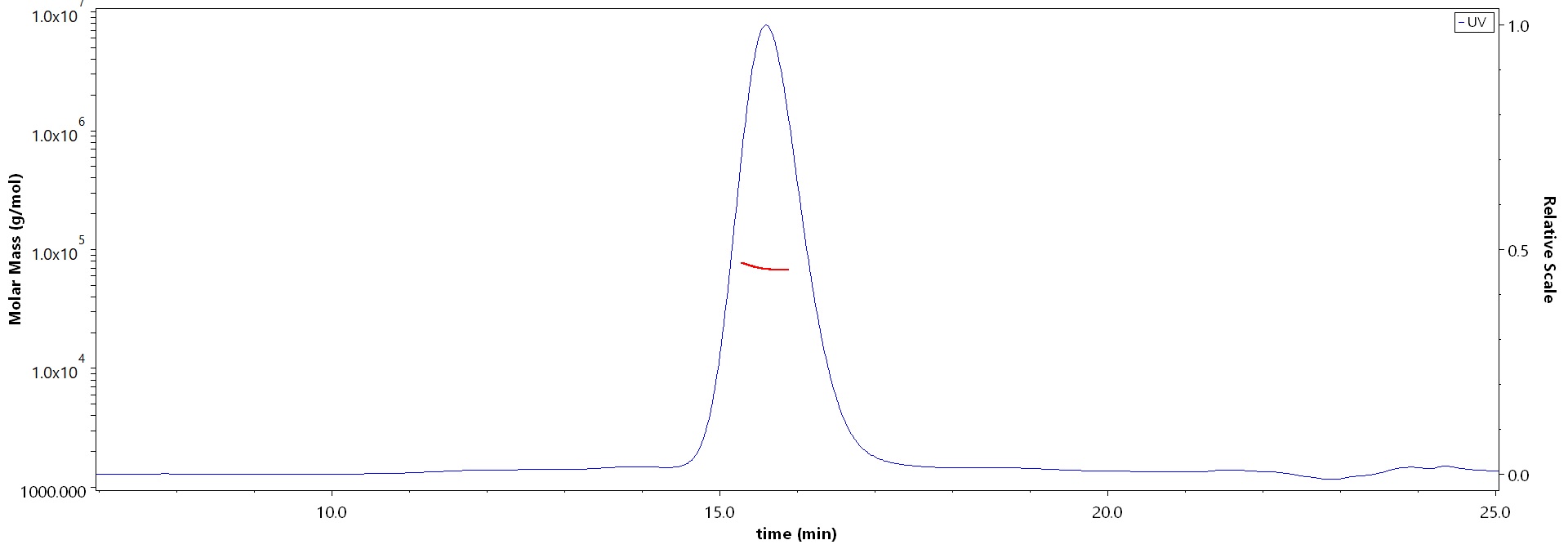
The purity of Cynomolgus Coagulation factor IX, His Tag (Cat. No. FIX-C52H9) is more than 90% and the molecular weight of this protein is around 55-75 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Anti-inhibitor coagulant complex (Baxalta) | Approved | Baxalta Incorporated | Feiba, Prothromplex TOTAL | United States | Hemophilia A; Hemophilia B | Baxalta Incorporated | 1979-12-21 | Blood Coagulation Disorders; Blood Coagulation Disorders, Inherited; Hemophilia A; Hemophilia B; Cardiovascular Diseases | Details | |
| Human prothrombin complex (Nanyue Biopharming) | Approved | Hunan Ziguang Huhan Nanyue Pharmaceutical Co Ltd | Mainland China | Hemorrhage | Hunan Ziguang Huhan Nanyue Pharmaceutical Co Ltd | 2019-09-05 | Hemophilia B; Hemorrhage | Details | ||
| Eptacog alfa (Novo Nordisk) | NN-007 | Approved | Novo Nordisk A/S | NiaStase, NovoSeven, 诺其 | EU | Factor VII Deficiency; Hemophilia A; Hemophilia B; Thrombasthenia | Novo Nordisk A/S | 1996-02-23 | Cerebral Hemorrhage; Spinal Diseases; Hemophilia A; Hemophilia B; Fibrosis; Hemorrhage; Stroke; Postpartum Hemorrhage; Factor VII Deficiency; Severe Dengue; Burns; Wounds and Injuries; Blood Loss, Surgical; Thrombasthenia; Acquired hemophilia | Details |
| Etranacogene dezaparvovec | AAV5-hFIX; AMT-061; CSL-222; AMT-060 | Approved | University College London, Uniqure Biopharma Bv | HEMGENIX | United States | Hemophilia B | Csl Behring | 2022-11-22 | Hemophilia B | Details |
| Human prothrombin complex (CSL Behring) | BE-1116 | Approved | Csl Behring Llc | Kcentra, Beriplex, Confidex | Blood Coagulation Disorders; Coagulation Protein Disorders; Heart Failure; Heart Diseases; Hemorrhage; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Wounds and Injuries | Details | ||||
| Freeze-dried activated human blood coagulation factor Ⅶ concentrate containing factor Ⅹ (Kaketsuken/KM Biologics) | MC-710 | Approved | Kaketsuken | Byclot | Japan | Hemophilia A | null | 2014-07-04 | Hemophilia A | Details |
| Human prothrombin complex concentrate (Guizhou Taibang Biological Products) | Approved | Guizhou Taibang Biological Products Co Ltd | Mainland China | Hypoprothrombinemias | Guizhou Taibang Biological Products Co Ltd | 2013-07-31 | Hypoprothrombinemias | Details | ||
| Prothrombinex-VF (CSL Behring) | Approved | Csl Behring Llc | Australia | Hemorrhage | Csl Behring Llc | 2014-10-01 | Hemorrhage | Details | ||
| Fidanacogene elaparvovec | SPK-9001; PF-06838435 | Approved | The Children'S Hospital Of Philadelphia | BEQVEZ | Canada | Hemophilia B | Pfizer Inc | 2024-01-03 | Hemophilia A; Hemophilia B | Details |
| Prothrombin complex concentrate (Sanquin) | Approved | Sanquin Plasma Products Bv | Cofact | Hemorrhage | Sanquin Plasma Products Bv | 2007-11-01 | Hemorrhage | Details | ||
| Emicizumab | RG-6013; ACE-910; RO-5534262 | Approved | Genentech Inc, Chugai Pharmaceutical Co Ltd | 舒友立乐, Hemlibra | United States | Hemophilia A | Genentech Inc | 2017-11-16 | Hemophilia A; Hemorrhage; von Willebrand Disease, Type 3 | Details |
| Factor XP Behring (CSL Behring) | Approved | Csl Behring Llc | Spain | Factor X Deficiency | Csl Behring Llc | 2015-09-01 | Factor X Deficiency | Details | ||
| Eptacog alfa biosimilar (AryoGen Biopharma) | Approved | Aryogen Biopharma | Iran | Factor VII Deficiency; Hemophilia A; Hemophilia B; Thrombasthenia | Aryogen Biopharma | 2014-01-01 | Hemophilia A; Hemophilia B; Factor VII Deficiency; Thrombasthenia | Details | ||
| Factor IX complex (Grifols) | Approved | Grifols Sa | Profilnine | United States | Hemophilia B | null | 1981-07-21 | Intracranial Hemorrhages; Hemophilia B | Details | |
| Eptacog beta | LR-769; rhFVIIa | Approved | Lfb Biotechnologies Sas, Revo Biologics Inc | Sevenfact | United States | Hemophilia A; Hemophilia B | Lfb Biotechnologies Sas | 2020-04-01 | Hemophilia A; Hemophilia B | Details |
| Human prothrombin complex (Octapharma) | Approved | Octapharma | Ocplex, Octaplex, Pronativ | Germany | Hemorrhage | Octapharma | 2003-03-14 | Hemorrhage; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Eptacog alfa biosimilar (International Biotech Center Generium) | GNR-001 | Approved | International Biotech Center Generium | Russian Federation | Hemophilia A | International Biotech Center Generium | 2015-01-01 | Hemophilia A | Details | |
| Human prothrombin complex (LFB) | Approved | Lfb Biotechnologies Sas | KANOKAD | France | Hemorrhage | Lfb Biotechnologies Sas | 2008-09-01 | Hemorrhage | Details | |
| Human prothrombin complex (Tonrol Biopharmaceuticals) | Approved | Tonrol Bio-Pharmaceutical Co Ltd | ProthoRAAS | Mainland China | Hemorrhage | Shanghai Raas Blood Products Co Ltd | 1996-01-01 | Hemophilia B; Hemorrhage | Details | |
| Human prothrombin complex (Guangdong Shuanglin) | Approved | Guangdong Shuanglin Bio-Pharmaceutical Co Ltd | Mainland China | Hemophilia B | Guangdong Shuanglin Bio-Pharmaceutical Co Ltd | 2023-05-12 | Hemophilia B | Details | ||
| Human prothrombin complex (Shanghai Xinxing) | Approved | Mainland China | Hemorrhage | Shanghai Xinxing Medicine Co Ltd | 1997-01-01 | Hemorrhage | Details | |||
| Human prothrombin complex (Sinopharm Shanghai Plasma) | Approved | Sinopharm Shanghai Plasma-Derived Biotherapies Co Ltd | Mainland China | Hemorrhage | Sinopharm Shanghai Plasma-Derived Biotherapies Co Ltd | 1997-01-01 | Hemorrhage | Details | ||
| Human prothrombin complex (Boya Bio-Pharmaceutical) | Approved | Boya Bio-Pharmaceutical Group Co Ltd | Mainland China | Hemorrhage | Boya Bio-Pharmaceutical Group Co Ltd | 2020-12-02 | Hemophilia B; Hemorrhage | Details | ||
| Human prothrombin complex (Guangdong Wellen) | Approved | Guangdong Wellen Pharmaceutical Co Ltd | Mainland China | Hemorrhage | Guangdong Wellen Pharmaceutical Co Ltd | 2020-10-21 | Hemophilia B; Hemorrhage | Details | ||
| Factor IX (Octapharma ) | Approved | Octapharma | EU | Hemophilia B | Octapharma | 2001-12-23 | Hemophilia B | Details | ||
| Human prothrombin complex(Hebei Daan Pharmaceutical) | Approved | Hebei Daan Pharmaceutical Co Ltd | Mainland China | Hemorrhage | Hebei Daan Pharmaceutical Co Ltd | 2020-01-01 | Hemophilia B; Hemorrhage | Details | ||
| Prothrombin complex concentrat (Taibang Biological Products) | Approved | Shandong Taibang Biological Products Co Ltd | Mainland China | Hemorrhage | Shandong Taibang Biological Products Co Ltd | 2014-09-30 | Hemorrhage; Antithrombin III Deficiency | Details | ||
| Human prothrombin complex (Hualan Biological Engineering) | Approved | Hualan Genetic Engineering Co Ltd | Mainland China | Blood Coagulation Disorders; Factor X Deficiency | Hualan Biological Engineering Inc | 1999-01-01 | Blood Coagulation Disorders; Hemophilia B; Factor X Deficiency | Details | ||
| Human prothrombin complex concentrate (Shanxi Kangbao Biological Products) | Approved | Shanxi Kangbao Biological Product Co Ltd | Mainland China | Hemorrhage | Shanxi Kangbao Biological Product Co Ltd | 2019-09-17 | Hemophilia B; Hemorrhage | Details | ||
| Anti-inhibitor coagulant complex (Baxalta) | Approved | Baxalta Incorporated | Feiba, Prothromplex TOTAL | United States | Hemophilia A; Hemophilia B | Baxalta Incorporated | 1979-12-21 | Blood Coagulation Disorders; Blood Coagulation Disorders, Inherited; Hemophilia A; Hemophilia B; Cardiovascular Diseases | Details | |
| Human prothrombin complex (Nanyue Biopharming) | Approved | Hunan Ziguang Huhan Nanyue Pharmaceutical Co Ltd | Mainland China | Hemorrhage | Hunan Ziguang Huhan Nanyue Pharmaceutical Co Ltd | 2019-09-05 | Hemophilia B; Hemorrhage | Details | ||
| Eptacog alfa (Novo Nordisk) | NN-007 | Approved | Novo Nordisk A/S | NiaStase, NovoSeven, 诺其 | EU | Factor VII Deficiency; Hemophilia A; Hemophilia B; Thrombasthenia | Novo Nordisk A/S | 1996-02-23 | Cerebral Hemorrhage; Spinal Diseases; Hemophilia A; Hemophilia B; Fibrosis; Hemorrhage; Stroke; Postpartum Hemorrhage; Factor VII Deficiency; Severe Dengue; Burns; Wounds and Injuries; Blood Loss, Surgical; Thrombasthenia; Acquired hemophilia | Details |
| Etranacogene dezaparvovec | AAV5-hFIX; AMT-061; CSL-222; AMT-060 | Approved | University College London, Uniqure Biopharma Bv | HEMGENIX | United States | Hemophilia B | Csl Behring | 2022-11-22 | Hemophilia B | Details |
| Human prothrombin complex (CSL Behring) | BE-1116 | Approved | Csl Behring Llc | Kcentra, Beriplex, Confidex | Blood Coagulation Disorders; Coagulation Protein Disorders; Heart Failure; Heart Diseases; Hemorrhage; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Wounds and Injuries | Details | ||||
| Freeze-dried activated human blood coagulation factor Ⅶ concentrate containing factor Ⅹ (Kaketsuken/KM Biologics) | MC-710 | Approved | Kaketsuken | Byclot | Japan | Hemophilia A | null | 2014-07-04 | Hemophilia A | Details |
| Human prothrombin complex concentrate (Guizhou Taibang Biological Products) | Approved | Guizhou Taibang Biological Products Co Ltd | Mainland China | Hypoprothrombinemias | Guizhou Taibang Biological Products Co Ltd | 2013-07-31 | Hypoprothrombinemias | Details | ||
| Prothrombinex-VF (CSL Behring) | Approved | Csl Behring Llc | Australia | Hemorrhage | Csl Behring Llc | 2014-10-01 | Hemorrhage | Details | ||
| Fidanacogene elaparvovec | SPK-9001; PF-06838435 | Approved | The Children'S Hospital Of Philadelphia | BEQVEZ | Canada | Hemophilia B | Pfizer Inc | 2024-01-03 | Hemophilia A; Hemophilia B | Details |
| Prothrombin complex concentrate (Sanquin) | Approved | Sanquin Plasma Products Bv | Cofact | Hemorrhage | Sanquin Plasma Products Bv | 2007-11-01 | Hemorrhage | Details | ||
| Emicizumab | RG-6013; ACE-910; RO-5534262 | Approved | Genentech Inc, Chugai Pharmaceutical Co Ltd | 舒友立乐, Hemlibra | United States | Hemophilia A | Genentech Inc | 2017-11-16 | Hemophilia A; Hemorrhage; von Willebrand Disease, Type 3 | Details |
| Factor XP Behring (CSL Behring) | Approved | Csl Behring Llc | Spain | Factor X Deficiency | Csl Behring Llc | 2015-09-01 | Factor X Deficiency | Details | ||
| Eptacog alfa biosimilar (AryoGen Biopharma) | Approved | Aryogen Biopharma | Iran | Factor VII Deficiency; Hemophilia A; Hemophilia B; Thrombasthenia | Aryogen Biopharma | 2014-01-01 | Hemophilia A; Hemophilia B; Factor VII Deficiency; Thrombasthenia | Details | ||
| Factor IX complex (Grifols) | Approved | Grifols Sa | Profilnine | United States | Hemophilia B | null | 1981-07-21 | Intracranial Hemorrhages; Hemophilia B | Details | |
| Eptacog beta | LR-769; rhFVIIa | Approved | Lfb Biotechnologies Sas, Revo Biologics Inc | Sevenfact | United States | Hemophilia A; Hemophilia B | Lfb Biotechnologies Sas | 2020-04-01 | Hemophilia A; Hemophilia B | Details |
| Human prothrombin complex (Octapharma) | Approved | Octapharma | Ocplex, Octaplex, Pronativ | Germany | Hemorrhage | Octapharma | 2003-03-14 | Hemorrhage; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Eptacog alfa biosimilar (International Biotech Center Generium) | GNR-001 | Approved | International Biotech Center Generium | Russian Federation | Hemophilia A | International Biotech Center Generium | 2015-01-01 | Hemophilia A | Details | |
| Human prothrombin complex (LFB) | Approved | Lfb Biotechnologies Sas | KANOKAD | France | Hemorrhage | Lfb Biotechnologies Sas | 2008-09-01 | Hemorrhage | Details | |
| Human prothrombin complex (Tonrol Biopharmaceuticals) | Approved | Tonrol Bio-Pharmaceutical Co Ltd | ProthoRAAS | Mainland China | Hemorrhage | Shanghai Raas Blood Products Co Ltd | 1996-01-01 | Hemophilia B; Hemorrhage | Details | |
| Human prothrombin complex (Guangdong Shuanglin) | Approved | Guangdong Shuanglin Bio-Pharmaceutical Co Ltd | Mainland China | Hemophilia B | Guangdong Shuanglin Bio-Pharmaceutical Co Ltd | 2023-05-12 | Hemophilia B | Details | ||
| Human prothrombin complex (Shanghai Xinxing) | Approved | Mainland China | Hemorrhage | Shanghai Xinxing Medicine Co Ltd | 1997-01-01 | Hemorrhage | Details | |||
| Human prothrombin complex (Sinopharm Shanghai Plasma) | Approved | Sinopharm Shanghai Plasma-Derived Biotherapies Co Ltd | Mainland China | Hemorrhage | Sinopharm Shanghai Plasma-Derived Biotherapies Co Ltd | 1997-01-01 | Hemorrhage | Details | ||
| Human prothrombin complex (Boya Bio-Pharmaceutical) | Approved | Boya Bio-Pharmaceutical Group Co Ltd | Mainland China | Hemorrhage | Boya Bio-Pharmaceutical Group Co Ltd | 2020-12-02 | Hemophilia B; Hemorrhage | Details | ||
| Human prothrombin complex (Guangdong Wellen) | Approved | Guangdong Wellen Pharmaceutical Co Ltd | Mainland China | Hemorrhage | Guangdong Wellen Pharmaceutical Co Ltd | 2020-10-21 | Hemophilia B; Hemorrhage | Details | ||
| Factor IX (Octapharma ) | Approved | Octapharma | EU | Hemophilia B | Octapharma | 2001-12-23 | Hemophilia B | Details | ||
| Human prothrombin complex(Hebei Daan Pharmaceutical) | Approved | Hebei Daan Pharmaceutical Co Ltd | Mainland China | Hemorrhage | Hebei Daan Pharmaceutical Co Ltd | 2020-01-01 | Hemophilia B; Hemorrhage | Details | ||
| Prothrombin complex concentrat (Taibang Biological Products) | Approved | Shandong Taibang Biological Products Co Ltd | Mainland China | Hemorrhage | Shandong Taibang Biological Products Co Ltd | 2014-09-30 | Hemorrhage; Antithrombin III Deficiency | Details | ||
| Human prothrombin complex (Hualan Biological Engineering) | Approved | Hualan Genetic Engineering Co Ltd | Mainland China | Blood Coagulation Disorders; Factor X Deficiency | Hualan Biological Engineering Inc | 1999-01-01 | Blood Coagulation Disorders; Hemophilia B; Factor X Deficiency | Details | ||
| Human prothrombin complex concentrate (Shanxi Kangbao Biological Products) | Approved | Shanxi Kangbao Biological Product Co Ltd | Mainland China | Hemorrhage | Shanxi Kangbao Biological Product Co Ltd | 2019-09-17 | Hemophilia B; Hemorrhage | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Mim8 | Mim8; NN7769; NNC0365-3769; NN-7769 | Phase 3 Clinical | Novo Nordisk A/S | Hemophilia A | Details |
| Eptacog Alfa Biosimilar(Aryogen Pharmed) | Phase 3 Clinical | Aryogen Pharmed Co | Hemophilia A; Factor VII Deficiency | Details | |
| CT-001 (Coagulant Therapeutics) | CT-001; CT001 | Phase 3 Clinical | Coagulant Therapeutics Corp | Pain; Postpartum Hemorrhage | Details |
| Human prothrombin complex concentrate (Sichuan Yuanda Shuyang Pharmaceutical) | Phase 3 Clinical | Sichuan Yuanda Shuyang Pharmaceutical Co Ltd | Blood Coagulation Disorders | Details | |
| BBM-H901 | BBM-H901 | Phase 3 Clinical | Belef BioMed (Shanghai) Co Ltd | Hemophilia B | Details |
| Human prothrombin complex concentrate (Harbin Shiheng Bioengineering Pharmaceutical) | Phase 3 Clinical | Harbin Pacific Biopharmaceutical Co Ltd | Hemophilia B | Details | |
| Human prothrombin complex (Lanzhou Lansheng Blood Products) | Phase 3 Clinical | Lanzhou Lansheng Blood Products Co Ltd | Hemophilia B | Details | |
| Recombinant human coagulation factor VIIa (Chengdu Rongsheng Pharmaceuticals) | Phase 3 Clinical | Chengdu Rongsheng Pharmaceuticals Co Ltd | Hemophilia A | Details | |
| Human prothrombin complex(Hunan Ziguang Huhan Nanyue Pharmaceutical) | Phase 3 Clinical | Hunan Ziguang Huhan Nanyue Pharmaceutical Co Ltd | Hemophilia B | Details | |
| aPCC-emicizumab | Phase 3 Clinical | Emory University | Hemophilia A | Details | |
| Dalcinonacog alfa | ISU304; ISU-304; CB-2679d | Phase 2 Clinical | Catalyst Bioscience | Hemophilia B | Details |
| BAX-335 | BAX-335; AskBio-009 | Phase 2 Clinical | Glaxosmithkline Plc, Asklepios BioPharmaceutical Inc, Bayer AG | Hemophilia B | Details |
| Factor VIIa-CTP | MOD-5014; MOD-5017; OPK88005 | Phase 2 Clinical | Opko Health Inc, Prolor Biotech | Blood Coagulation Disorders, Inherited; Hemophilia A; Hemophilia B | Details |
| FT-004 | FT-004; FT004 | Phase 2 Clinical | Frontera Therapeutics (Shanghai) Co Ltd | Hematologic Diseases; Hemophilia B | Details |
| ZS-801 | ZS801; ZS-801 | Phase 2 Clinical | Hemophilia B | Details | |
| ANB-002 | ANB-2; ANB-002 | Phase 2 Clinical | Biocad | Hemophilia B | Details |
| VGB-R04 | VGB-R04 | Phase 2 Clinical | Shanghai Vitalgen BioPharma Co Ltd | Hemophilia B | Details |
| Eptacog alfa biosimilar (Valin Technologies) | Phase 1 Clinical | Valin Technologies | Hemophilia A | Details | |
| Marzeptacog alfa | CB-813; CB-813d; PF-5280602; PF-05280602 | Phase 1 Clinical | Catalyst | Hemophilia A; Hemophilia B | Details |
| AAV2-hFIX16 | AAV2-hFIX16 | Phase 1 Clinical | Spark Therapeutics Inc | Hemophilia B | Details |
| SS-327 | GenSciences-327; 注射用培重组人凝血因子IX-Fc融合蛋白; SS327 | Phase 1 Clinical | Zhengzhou Shengsi Biotechnology Co Ltd, Furen Pharmaceutical Group Co Ltd | Hemophilia B | Details |
| JWK-004 | JWK004; JWK-004 | Phase 1 Clinical | Chengdu Genevector Therapeutics Co Ltd | Hemophilia B | Details |
| Human prothrombin complex (National Drug Group Wuhan Blood) | Phase 1 Clinical | Hemorrhage | Details | ||
| YUVA-GT-F901 | YUVA-GT-F901 | Phase 1 Clinical | Novartis Pharma Ag | Hemophilia B | Details |
| Mim8 | Mim8; NN7769; NNC0365-3769; NN-7769 | Phase 3 Clinical | Novo Nordisk A/S | Hemophilia A | Details |
| Eptacog Alfa Biosimilar(Aryogen Pharmed) | Phase 3 Clinical | Aryogen Pharmed Co | Hemophilia A; Factor VII Deficiency | Details | |
| CT-001 (Coagulant Therapeutics) | CT-001; CT001 | Phase 3 Clinical | Coagulant Therapeutics Corp | Pain; Postpartum Hemorrhage | Details |
| Human prothrombin complex concentrate (Sichuan Yuanda Shuyang Pharmaceutical) | Phase 3 Clinical | Sichuan Yuanda Shuyang Pharmaceutical Co Ltd | Blood Coagulation Disorders | Details | |
| BBM-H901 | BBM-H901 | Phase 3 Clinical | Belef BioMed (Shanghai) Co Ltd | Hemophilia B | Details |
| Human prothrombin complex concentrate (Harbin Shiheng Bioengineering Pharmaceutical) | Phase 3 Clinical | Harbin Pacific Biopharmaceutical Co Ltd | Hemophilia B | Details | |
| Human prothrombin complex (Lanzhou Lansheng Blood Products) | Phase 3 Clinical | Lanzhou Lansheng Blood Products Co Ltd | Hemophilia B | Details | |
| Recombinant human coagulation factor VIIa (Chengdu Rongsheng Pharmaceuticals) | Phase 3 Clinical | Chengdu Rongsheng Pharmaceuticals Co Ltd | Hemophilia A | Details | |
| Human prothrombin complex(Hunan Ziguang Huhan Nanyue Pharmaceutical) | Phase 3 Clinical | Hunan Ziguang Huhan Nanyue Pharmaceutical Co Ltd | Hemophilia B | Details | |
| aPCC-emicizumab | Phase 3 Clinical | Emory University | Hemophilia A | Details | |
| Dalcinonacog alfa | ISU304; ISU-304; CB-2679d | Phase 2 Clinical | Catalyst Bioscience | Hemophilia B | Details |
| BAX-335 | BAX-335; AskBio-009 | Phase 2 Clinical | Glaxosmithkline Plc, Asklepios BioPharmaceutical Inc, Bayer AG | Hemophilia B | Details |
| Factor VIIa-CTP | MOD-5014; MOD-5017; OPK88005 | Phase 2 Clinical | Opko Health Inc, Prolor Biotech | Blood Coagulation Disorders, Inherited; Hemophilia A; Hemophilia B | Details |
| FT-004 | FT-004; FT004 | Phase 2 Clinical | Frontera Therapeutics (Shanghai) Co Ltd | Hematologic Diseases; Hemophilia B | Details |
| ZS-801 | ZS801; ZS-801 | Phase 2 Clinical | Hemophilia B | Details | |
| ANB-002 | ANB-2; ANB-002 | Phase 2 Clinical | Biocad | Hemophilia B | Details |
| VGB-R04 | VGB-R04 | Phase 2 Clinical | Shanghai Vitalgen BioPharma Co Ltd | Hemophilia B | Details |
| Eptacog alfa biosimilar (Valin Technologies) | Phase 1 Clinical | Valin Technologies | Hemophilia A | Details | |
| Marzeptacog alfa | CB-813; CB-813d; PF-5280602; PF-05280602 | Phase 1 Clinical | Catalyst | Hemophilia A; Hemophilia B | Details |
| AAV2-hFIX16 | AAV2-hFIX16 | Phase 1 Clinical | Spark Therapeutics Inc | Hemophilia B | Details |
| SS-327 | GenSciences-327; 注射用培重组人凝血因子IX-Fc融合蛋白; SS327 | Phase 1 Clinical | Zhengzhou Shengsi Biotechnology Co Ltd, Furen Pharmaceutical Group Co Ltd | Hemophilia B | Details |
| JWK-004 | JWK004; JWK-004 | Phase 1 Clinical | Chengdu Genevector Therapeutics Co Ltd | Hemophilia B | Details |
| Human prothrombin complex (National Drug Group Wuhan Blood) | Phase 1 Clinical | Hemorrhage | Details | ||
| YUVA-GT-F901 | YUVA-GT-F901 | Phase 1 Clinical | Novartis Pharma Ag | Hemophilia B | Details |
This web search service is supported by Google Inc.